Telomere-led rapid prophase movements of the chromosomes (RPMs) have been proposed to move chromosomes relative to one another, helping establish homologous interactions during pairing, resolve chromosome entanglements and regulate chiasma placement. Dramatic prophase movements have been observed in a wide range of organisms including mouse.
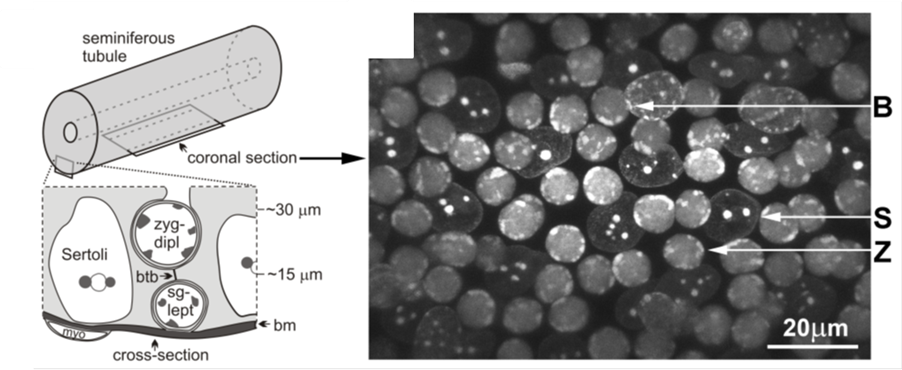
Figure 4. Diagram showing the location of the indicated cell types and their approximate distances from the basement membrane (bm) of the seminiferous tubule as viewed in cross-section. The position of a coronal section, as shown in C and used to acquire time-lapse images, is also indicated.
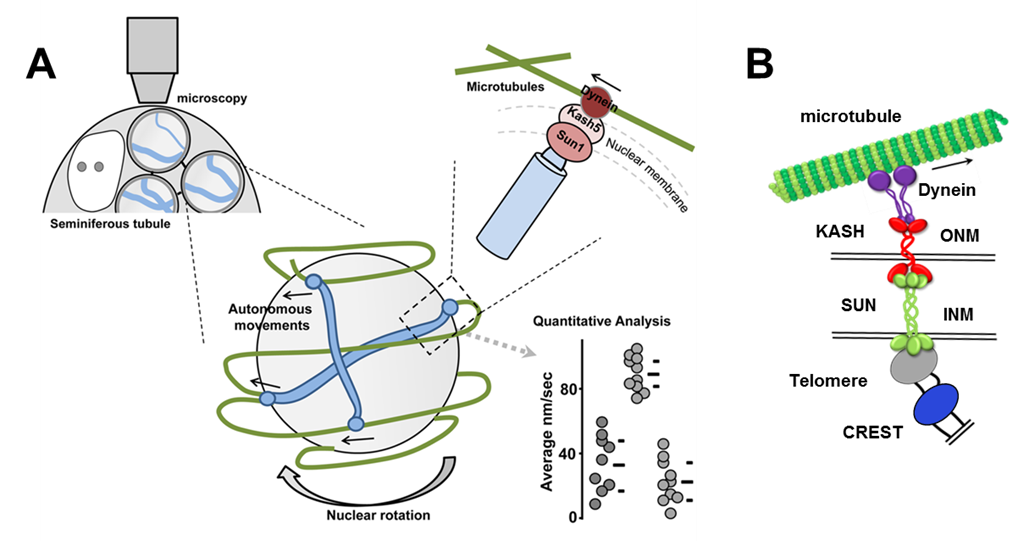
Figure 5. Mechanism of rapid telomere movements in mouse spermatocytes. A. Scheme shows the strategy used to record and score rapid telomere chromosome movements in mouse spermatocytes. B. Proposed model for meiotic chromosome telomere-nuclear envelope attachment and connection to dynein on microtubules.
We recently employed 3D fluorescence time-lapse microscopy in intact seminiferous tubules supplemented with customized visualization, and in silico analysis and simulation (Figure 4). This approach proved to have the sensitivity to reveal changes in RPM characteristics during meiotic progression (movies 1-4), and differences in RPMs brought about by defects in recombination and synapsis. Our study reveals that in mouse spermatocytes RPMs are result of a combination of nuclear rotation and chromosome autonomous movements (Figure 5A and movie 5). The chromosome autonomous movements are consistent with a mechanism by which telomeres move along microtubule tracks and can change directions at intersections of the tracks (Figure 5B). Our work in mouse spermatocytes has revealed the mechanism supporting active prophase chromosome movements. This involves dynein on cytoskeletal microtubules that originate the forces generating the movements. A particularly conserved aspect of chromosome movements is the protein complexes that bridge telomeres to the cytoskeleton and provide the molecular connections that can transduce forces generated in the cytoplasm to the end of the chromosomes (Figure 5B). In the mouse, the SUN1 and KASH5 proteins are localized to the inner and outer nuclear membrane of the nuclear envelope, respectively, and physically interact with each other connecting the internal regions of the nuclear envelope with the cytoskeleton.
Movies
Movie 1 – Leptotene
Movie 2 – Bouquet
Movie 3 – Zygotene
Movie 4 – Pachytene
Movie 5 – Rotation Movement